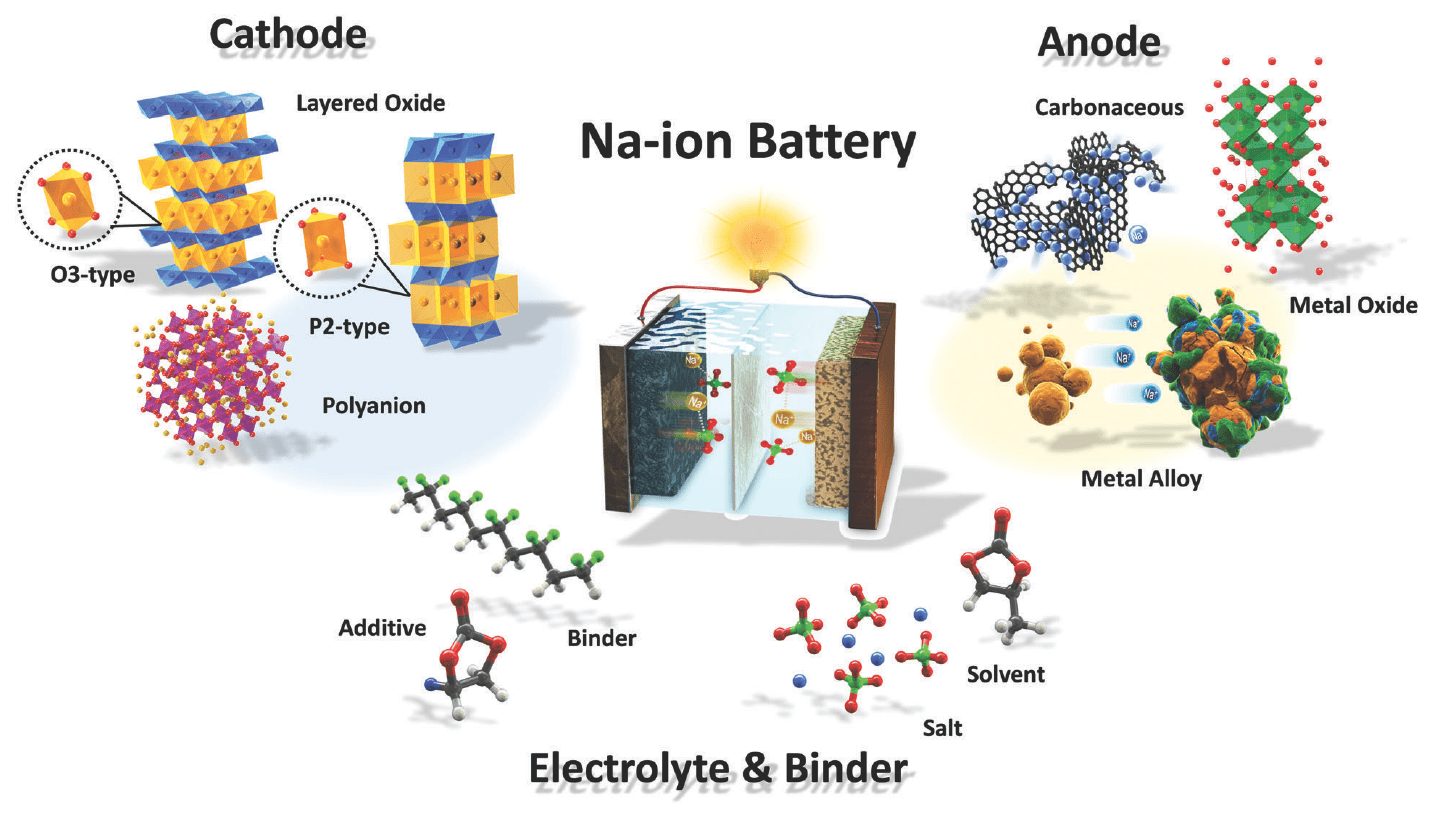
Vhiki nyowani uye bhatiri nyowani: Na-ion (sodium-ion), yakafanana mumaparamita kune Li-ion, asi kazhinji inochipa.
Vatsvagiri veWashington State University (WSU) vakagadzira bhatiri re "munyu wakawedzera" rinoshandisa sodium pachinzvimbo che lithium. Sodium (Na) ndeyeboka realkali metals, ine makemikari akafanana, saka masero anobva pairi ane mukana wekukwikwidzana neLi-ion. Zvirinani mune mamwe maapplication.
Na-ion mabhatiri: zvakachipa zvakanyanya, zvishoma zvakaderera kune lithium-ion, pasi pekutsvagisa
Sodium chimwe chezvinhu zviviri mu sodium chloride (NaCl) tafura yemunyu. Kusiyana ne lithium, inowanikwa mukuwanda zvese mumadhipoziti (dombo remunyu) uye mumakungwa nemumakungwa. Nokudaro, masero eNa-ion anogona kunge akachipa kakawanda kupfuura masero e-lithium-ion, uye nenzira, anofanirwa kuvakwa achishandisa zvinhu zvakafanana uye zvigadziro semasero e-lithium-ion.
Kushanda pamasero eNa-ion kwakaitwa makore angangoita 50-40 apfuura, asi akazoregedzwa. Iyo sodium ion yakakura kudarika lithium ion, saka zvinhu zvine dambudziko nekuchengetedza mutero wakakodzera. Chimiro chegraphite - chakakura zvakakwana kune lithium ions - chakazove chakanyanya kuomarara kune sodium.
Tsvagiridzo yakaona kudzokazve mumakore mashoma apfuura sezvo kudikanwa kwemasero emagetsi ekushandisa zvakare kwakakwira. WSU masayendisiti akagadzira sodium-ion bhatiri iyo inofanirwa kuchengetedza huwandu hwesimba rakafanana neiyo yakafanana lithium-ion bhatiri. Uye zvakare, bhatiri rakapona 1 kutenderera kutenderera uye rakachengeta zvinopfuura 000 muzana yezvayakaita yekutanga (yekutanga).
Ose maviri aya paramita anoonekwa se "akanaka" munyika yelithium-ion mabhatiri. Nekudaro, kune zvinhu zvine sodium ions, kutevedzera mamiriro kwakave kwakaoma nekuda kwekukura kwemakristasi esodium pane cathode. Nokudaro, zvakasarudzwa kushandisa chidziviriro chesimbi oxide uye electrolyte ine yakanyungudutswa sodium ions, iyo yakagadzirisa chimiro. Akabudirira.
Iyo yakaderera yeNa ion cell ndiyo yakaderera simba density, izvo zvinonzwisisika kana saizi yelithium uye maatomu esodium akatariswa. Nekudaro, kunyangwe nyaya iyi ichigona kunetsa mumotokari yemagetsi, haina kukanganisa zvachose kuchengetedza kwesimba. Kunyange kana Na-ion ikatora kaviri nzvimbo yakawanda se lithiamu-ion, mutengo wayo wakapetwa kaviri kana katatu wakaderera uchaita kuti sarudzo ionekwe.
Iyi chete ndiyo yekutanga mumakore mashoma ...
Izvi zvinogona kukufadza:
